Abstract:
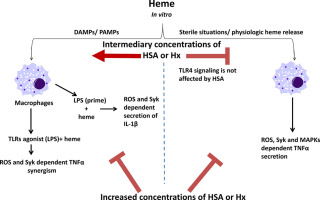
Free heme released from hemoglobin contributes to exacerbated inflammation and tissue damage in hemolytic diseases. While a moderate level of free heme does not cause intravascular inflammation by itself, its presence during infection greatly enhances inflammation. Although specific serum proteins have been found to affect heme-induced inflammation, the selective contribution of serum proteins inhibiting macrophage activation by heme or, conversely, amplifying the production of cytokines by macrophages stimulated with heme and microbial molecules, is poorly defined. Here we identified a serum fraction containing proteins with >50 KDa which was capable of inhibiting heme-stimulated TNF production and capable of enabling TNF production under conditions of a heme-LPS synergy. The inhibition of heme-induced TNF production was mimicked by Hemopexin (Hx), human serum albumin (HSA), serum from Hx-knockout mice, and less efficiently by serum from albumin-knockout mice, but not by serum LDL. Hx and HSA inhibited heme-induced ROS generation, MAPK/ Syk phosphorylation and cell death. However, Hx and HSA each also promoted the synergistic relationship between heme and LPS upon TNF production. Serum from Hx-knockout mice was fully capable of enabling this synergy, while serum from albumin-knockout mice was less efficient to promote TNF production under these conditions. Low concentrations of HSA mimicked the ability of serum to enable heme-stimulated IL-1β production after LPS priming, while high concentrations inhibited it. Together, our findings indicate how heme inflammatory effects are restrained in the blood upon sterile hemolysis, yet exacerbate inflammation in the presence of microbes. Moreover, it is interesting to note that opposing effects of serum proteins on heme-induced macrophage activation were selected through evolution, with both effects exerted by Hx and albumin.
Figure: